Abstract
Introduction: After Rituximab (R) plus cyclophosphamide, doxorubicin, and prednisone (R-CHOP) induction therapy (IT), the use of R as maintenance (RM) has improved the outcome of patients with follicular lymphoma (FL). R plus Bendamustine (R-B) has demonstrated excellent efficacy in this setting, but the role of RM is not clear. Therefore, the aim of this retrospective study was to compare, in terms of efficacy and toxicity, both R-based regimens followed by RM for patients with untreated advanced-stage follicular FL.
Methods: We retrospectively assessed patients with FL from 17 different GELTAMO centres, treated with either R-B or R-CHOP as first line therapy followed by RM. The outcome and toxicity were evaluated.
Results: Of total of 434 patients with FL treated with R-CHOP or R-B, 67 were excluded because they had not received RM despite not having had toxicity/death or progression. Finally, 367 patients were included,146 received R-B and 221 treated with R-CHOP. Patients with grade 3A FL (R-CHOP 57 (28%) R-B 13 (10%), p= 0.001) or age £ 60 (median age 57 (22-84) for RCHOP and 62 (31-101) for R-B, p= 0.002) significantly received more R-CHOP. FLIPI 3-5 was also more frequent with R-CHOP (103 [49%] for RCHOP and 38 [36%] for R-B, p= 0.048). During IT, G-CSF prophylaxis and cytopenias were more frequent with R-CHOP, however infections were similar (R-B 22 [24,2%] vs R-CHOP 51 [25,4%], p=0.8), but hospitalizations were more frequent with R-CHOP (R-B 17 [12%] vs R-CHOP 44 [21%], p=0.04).
One hundred and nine patients (73%) in the R-B group and 193 (87%) in the R-CHOP group completed the planned RM. Neutropenia (R-B 60 [36%] vs R-CHOP 449 [15%], p=0.001) and infections (R-B 36 [27%] vs R-CHOP 26 [13%], p=0.002) were most common with RM after R-B. RM discontinuation was more frequent in the R-B group (p=0.002), the most common reasons were cytopenias and infections, while with R-CHOP was progression. We did not find differences in the incidence of secondary neoplasms.
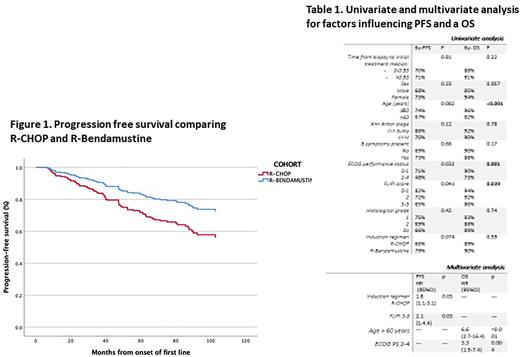
Overall and complete response (OR/CR) rates at the end of IT were higher for R-B (OR/CR 143 [98%]/123 [84%] when compared with 212 [93%]/161 [73%] in R-CHOP (p=0.02)). The frequency of progression in the first 24 months (POD 24) was similar for both groups (14 [10%] for R-B and 24 [11%] for R-CHOP). Relapses were more frequent with R-CHOP (48 [22%], R-B 14 [10%], p=0.005)). After a median follow-up of 78 months (5-239) (66 [9-122] for R-B and 89 [5-239] for R-CHOP), 6-year rate of progression-free survival was 79% (95%CI: 71-86) for R-B vs 66% (95%CI: 59-72) for R-CHOP (p= 0.074) and 6-year-overall survival was 90% (95%CI: 85-96) for R-B vs. 89% (95%CI: 85-94) for R-CHOP (p= 0.59). Multivariate analysis identified FLIPI 3-5 (HR 2.1; p=0.037) and induction regimen R-CHOP (HR 1.8; p=0.03) independently associated with PFS, and age >60 years (HR 6.6; p<0.001) and ECOG PS 2-4 (HR 3.3; p=0.004) with OS (Table 1). Figure 1 shows multivariate influence of induction regimen on PFS.
Conclusion: In this series, R-B followed by RM showed superior efficacy compared with R-CHOP followed by RM in untreated advanced-stage FL. RM after R-B was associated with more toxicity, especially neutropenia and infections.
Disclosures
Bastos-Oreiro:Roche: Consultancy, Research Funding, Speakers Bureau; INCYTE: Consultancy, Speakers Bureau; JANSSEN: Speakers Bureau; NOVARTIS: Speakers Bureau; KITE/GILEAD: Consultancy, Honoraria. Jimenez-Ubieto:Novartis: Consultancy. De La Fuente:Daiichi Sankyo: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau. Cordoba:Pfizer: Consultancy, Speakers Bureau; GenMab: Consultancy; Celgene: Consultancy, Honoraria; Takeda: Consultancy; Gilead: Honoraria; Kite: Consultancy; Bristol Myers: Research Funding. González de Villambrosia:Takeda: Honoraria; Janssen: Honoraria; Incyte: Honoraria; EusaPharma: Honoraria. Salar:Incyte: Speakers Bureau; Gilead: Research Funding; Janssen: Honoraria, Speakers Bureau; BMS/Celgene: Honoraria; Beigene: Honoraria; Abbvie: Research Funding; Roche: Research Funding, Speakers Bureau. Sancho:Bristol Myers Squibb: Honoraria; Miltenyi Biomedicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eli Lilly & Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kern Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal